6. AIR COMBUSTION, RUSTING AND FIRE FIGHTING
AIR
This is a homogeneous colorless mixture of different gases in the atmosphere.
Composition of gases in air
The gases include: oxygen, nitrogen, carbon dioxide, and noble gas. Air contains water vapor and dust particles. However, they are not usually considered part of air.
The table below shows the percentage composition of gases.
|
Gas |
% composition |
|
Nitrogen |
78% |
|
Oxygen |
21% |
| Noble gases |
0.94% |
|
Carbon dioxide |
0.03% |
|
Water vapor |
0-4% |
NB:The percentage composition of water vapor is generally caused by;
•Weather
•Geographical location
Test for gases in air.
-It is possible to test for the presence of some of the gases that are found in air.
Carbon dioxide test
-Lime water on the watch glass turns milky after a few days when left exposed. The conversion of the lime water from colourless to milky color indicates that there is carbondioxide present in the air.
Oxygen test
- It relights a glowing splint i.e when oxygen is collected in a gas jar and a piece of wood that has been lighted with fire is also placed within the jar, its going to be relighted because oxygen supports burning. Or copper turns to black on heating because it reacts with oxygen.
Water
-White anhydrous copper (ii) sulphate on watch glass turns blue when left exposed.This is due the presence of water in air.
Combustion
-This is a chemical reaction that involves the burning of a substance in the presence of oxygen then produces energy and light.
Combustible
-Is a material that catch fire and burn easily, combustion takes place in the open place such as fire or in a closed system such as a car engine.
Application of combustion in real life
Areas where combustion is applied;
|
Area |
Application |
|
Industries |
|
|
Domestic |
|
|
Laboratory |
|
FIRE FIGHTING
Fire - is the process in which ignited material combine with oxygen to give light, heat and a flame.
Fire fighting - is the extinguishing of harmful fire.
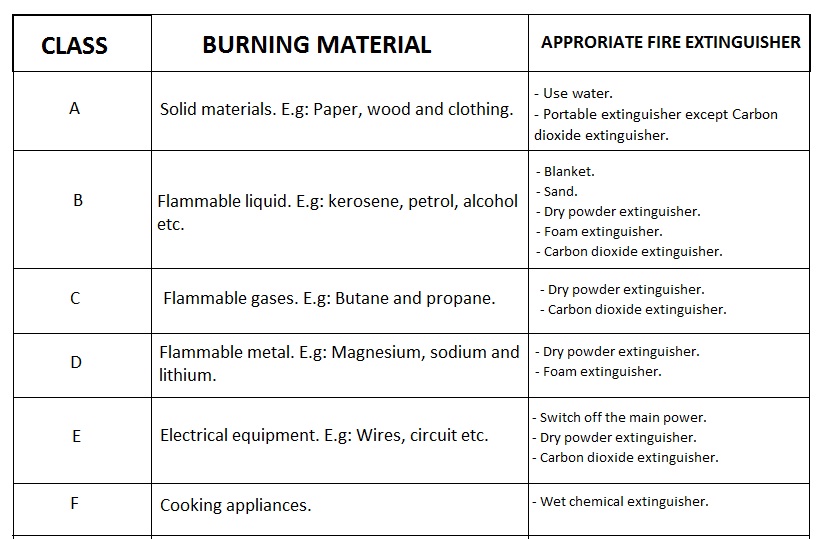
Classification of fire
Fire can be classified according to the types of materials burning hence each type of fire will need appropriate fighting technique and equipment.
The table below shows classification of fires and the appropriate extinguishers
Components needed to start fire
For a fire to burn the following components are required in suitable proportions;
-
Source of ignition heat
-
Air (oxygen)
-
Fuel
-The above three components are called fire triangles. If any of them is missing no fire will continue burning.
NB: during the fire fighting at least one component is eliminated to cut off the fire especially air.
 .
.
FIRE EXTINGUISHERS;
Is an active fire protection device used to extinguisher control small fire often in emergency situation.
> Portable fire extinguisher is an extinguisher that can be easily moved from one place to another.
TYPES OF FIRE EXTINGUISHERS
There are four common types of portable fire extinguishers namely:
-
Dry powder extinguishers;
Contain fire sodium bicarbonate powder pressurized by nitrogen.Extinguish the fire by separating the fuel from the oxygen element or by removing the heat element of the fire triangle.
-
Foam extinguishers;
Contains proteins and fluoroproteins.extinguisher fire by taking a way the heat element of the fire triangle .From agents also separate the oxygen element from the other element.
-
Water extinguisher;
Contain ordinary tap water pressurized air, extinguish fire by taking away the heat element of the fire triangle.
-
Carbon dioxide extinguisher;
Contain CO2, a non flammable gas, and are highly pressurized air. Extinguish fire by taking a way the oxygen element of the fire triangle and also be removing the heat with very cold discharge.
Using fire extinguishes
The fire extinguishers should be used based on the following; "PASS"
-
PULL - The safety pin from the handle. The pin is located at the top of the fire extinguisher. Once removed, it releases the locking mechanism, allowing you to discharge the extinguisher.
-
AIM - The extinguisher nozzle at the base of the fire. As explained this removes the sources or fuel of the fire. Keep yourself low.
- SQUEEZE - The handle slowly to discharge the agent. Letting go of the handle will stop the discharge, so keep it held down.
- SWEEP - Sweep side to side over the fire until expended. The sweeping motion helps to extinguish the fire. Stand few meters back from the fire.
RUSTING
Rust: is the reddish brown oxide of iron formed by the action of moisture and oxygen on the mental surface. It consists mainly of hydrated iron (III) oxide (Fe2O3.H2O) and iron (III) hydroxide (Fe(OH)3)
RUSTING: Is the process in which iron turns into iron oxide
Rusting process ; steps are the follows
(i) Fe →Fe2+ + 2e-
(ii) 2e- +½O2 +H2O →2OH
>The hydroxide ion react with iron (Fe²+)and more dissolved oxygen to from iron (iii) oxide usually in hydrated form .
(III) Fe2+ + 2OH-→ Fe (OH) 2
2Fe (OH) 2 +1/2O2 + XH2O Fe2O3.XH2O+2H2O
This is a chemical process that occurs in iron or steel forming a red brown coating on the metal.
Conditions for rusting
There are three conditions necessary for rusting and these are;
-
Iron steel
-
Water moisture
-
Air oxygen
Methods of preventing rusting
•Painting-Is the coating of items with a special pigment point that are made of iron and usually painted to make the last long.
•Oiling-Is the coating of iron with oil .Some machine parts cannot be protected with painting so they use oil.
•Tin plating- Is the coating of iron with tin. Tin cans are actually of steel, but inside of them can be coated with a thin layer of tin to make them suitable for carrying foods.
•Galvanization- Is the coating of iron or steel with zinc.
•Use of silica gel- This is a substance in the form of grate to absorb moisture in a small bag of silica gel is put inside carrying the instrument so as to absorb at moisture that may be there.
•Use of plastics - Parts of some machines or instruments are coated with plastic to ensure that they do not rust.
REVIEW QUESTION
1. Define the following
a/ (i) Rusting
(ii) Rust
(iii) Air
(iv) Combustion
(v) Fire
(vi) Fire fighting
b/ Mention the components of air and their percentage
2.(a) Mention classes of fire ,their burning materials and appropriate fire extinguisher.
(b) What are the components needed to start fire .
(c) What are the conditions necessary for ion to rust.
3.(a) Classify the types of extinguisher according to the chemical they contain.
(b) Explain with equation how rusting occur .
(c) Mention the methods of preventing rusting.